QUALITY
Establishment and implementation of Quality Systems to comply with ISO 13485, MDR, IVDR, MDSAP & FDA-QSR; Establishing eQMS; Execution of quality audits and mock FDA audits; Vast experience with FDA compliance requirements and implementation; QA Engineering tasks, V&V support; Remediation projects.
REGULATION
Development of regulatory strategies; Guidance in the implementation of regulatory requirements through the Design and Market Phases; Regulatory Submissions (FDA, CE, Global Regulations); Support ongoing regulatory tasks such as risk management, complaint management, CAPA & ECO processes; SW QA; Cybersecurity; Information Privacy, registration & listing and more
CRO - CLINICAL STUDIES
Full CRO services: Submission Packages to IRB; Clinical Investigation Plans (SIP, Study Protocol); Investigators Brochure (IB); Informed Consent form (IC); Case
Report Form (CRF); Management & Conduct of clinical studies from submission through initiation and monitoring until study closure; Development of EDC/eCRF &
eTMF solutions.
INFORMATION SECURITY&PRIVACY
In the era of connected care, patient safety extends beyond physical devices to the data they generate and cybersecurity is now a critical regulatory requirement. We guide medical device and digital health companies through the complex landscape of global cybersecurity and privacy regulations, ensuring your technology remains compliant, secure, and trusted.
At HaMaDa, we are the trusted leaders in the field of medical device consulting. With a dedicated team of experts and a global perspective, we provide crucial services to bring your medical innovations to life.

STRATEGY
- RA Strategy
- QA Strategy
- Clinical Strategy
- V&V Planning
- Market Strategy
- Publication Strategy

DESIGN PHASE
- Device Requirements
- RMF (Risk Management)
- Usability
- Product Validation
- DHF
- VMP, V&V
- SDLC
- Cyber Security
- Information Privacy

MANUFACTURING
- Transfer To Production
- CAPA
- ECO
- DMR, DHR
- IQ, OQ, PQ
- Agreements
- Subcontractors
- Turn Key

QUALITY SYSTEM
- Gap Analysis
- Establishment
- Implementation
- Training
- Audits
- e-QMS
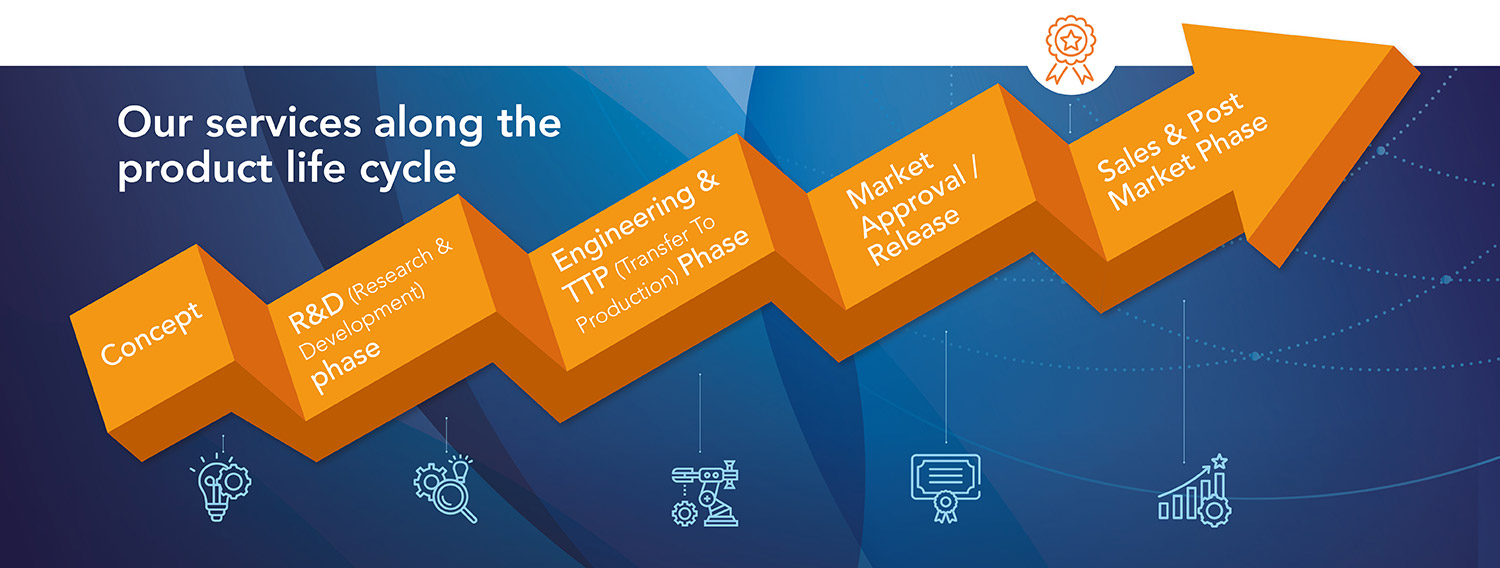

CRO
- Submission File
- Clinical Trial Conduct
- Clinical Trial Management (CIP, ICF, IB; eTMF, SOPs, CSRs)
- Clinical Writing (synopsis, protocol, articles)
- Monitoring
- Site Inspection
- Study Management
- EDC / eCRF

REGULATORY SUBMISSIONS
- Technical Documentation
- FDA (PMA, 510k, Denovo)
- Standard & Guidances
- MDR / IVDR
-
Medical Writing
(PMS, CER, PSUR) - Latam, Asia, Amar

AUDITS
- ISO 13485
- ISO 27001
- MDSAP
- MOCK FDA
- MDR / IVDR
- Clinical Trial Audit
- ISO 14155

SALES PHASE
- PMS
- Labeling, User Manual
- Publications
- Promotional Material
- PRRC
- Economic operator i.e. distributor, Importer
TRUSTED BY






















































