HaMaDa Medical Devices and Life Science Consulting will guide you through the regulatory, Medical Writing, Clinical Studies and Quality Assurance (QA) maze.
Our team of highly qualified professionals stays fully informed on the latest global regulations and standards, allowing you to concentrate on your core business with the confidence that you are receiving the essential expertise and support you need.
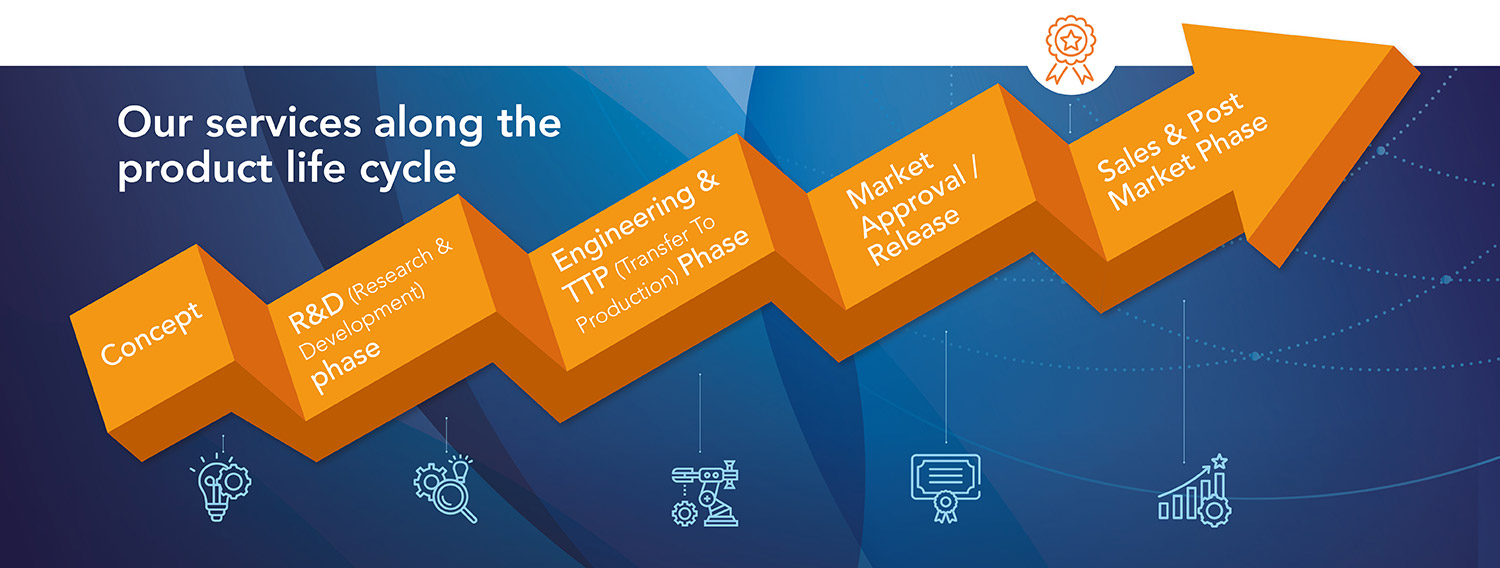
- Planning: Regulatory strategy and Regulatory plans.
- Ongoing regulatory support for the timely approval of products marketed globally including the USA, Europe, Canada, Israel, Australia, Brazil, and other regions.
- Manage the communication with regulatory authorities.
- Global Regulatory & Quality Consulting for Medical Device, combination products and IVD Companies.
- MDR documentation such as CEP/CER, PEP/PER, PMS, PSUR, PMCF and more.
- Publications.
- Protocols & Reports.
- Establishing, implementing, maintenance and auditing quality systems.
- Compliance with Quality system requirements such as the US FDA QSR (21 CFR 820); ISO 13485, MDSAP.
- Labeling Review and Development.
- Develop clinical study protocols.
- Prepare Study Submission Files including communication with Ethical Committee, Ministry of Health and other erlevant bodies.
- All aspects of study conduct according to Good Clinical Practices (GCP).
- Develop EDC/eCRF & eTMF.
Information Security & Privacy
- ISO 27001 Gap analysis, ISMS implementation, and maintenance for global security certification.
- HIPAA Compliance assessment, policy implementation, and PHI protection for US markets.
- SOC 2 Audit readiness, control mapping, and report preparation for trust services.
- GDPR (EU) DPO services, data impact assessments, and privacy implementation for Europe.
- Israel PPL Database registration and security regulation implementation for local compliance.
- AI Governance (ISO 42001) Risk management frameworks and compliance strategies for the EU AI Act.

